ZEVO™ ANTERIOR CERVICAL PLATE SYSTEM
Medtronic Press Release
May 5, 2015
 Medtronic plc (NYSE: MDT) announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the ZEVO™ Anterior Cervical Plate System. This system is now commercially available for the treatment of cervical degenerative disc disease, trauma, tumors, deformity, pseudoarthrosis, and/or failed previous fusions.
Medtronic plc (NYSE: MDT) announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the ZEVO™ Anterior Cervical Plate System. This system is now commercially available for the treatment of cervical degenerative disc disease, trauma, tumors, deformity, pseudoarthrosis, and/or failed previous fusions.
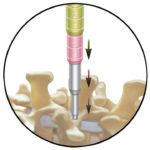
The ZEVO System represents Medtronic’s latest technology for anterior cervical discectomy with fusion (ACDF) procedures. It features shorter plating options coupled with hyper-screw angulations, allowing the physician to select the smallest possible plate for the patient’s individual surgical needs while securing the plate as far as possible from the adjacent disc.
"Versatility is important in ACDF procedures, and the ZEVO™ Anterior Cervical Plate System was designed to give surgeons more options,” said Doug King, president of the Spinal business and senior vice president of Medtronic. “This system represents Medtronic’s commitment to responding to surgeon needs, and innovating in ways that improve patient treatment and care.”
Literature shows this technique may lower the incidence of Adjacent Level Ossification. Development (ALOD) or bone growth next to the treated level. Additionally, the ZEVO System features lower-profile plates with minimal thickness (1.9mm and 2.1mm) while increasing the stability of the construct for the 4-5 level options.

"Improved, thinner plates are important in ACDF procedures," said Dr. Richard Hynes, spine surgeon at The B.A.C.K. Center in Melbourne, Florida. "ZEVO embodies these characteristics, with the added benefits of hyper screw angulations, which can be directed away from the spinal cord.”
About the ZEVO™ Anterior Cervical Plate System
The ZEVO™ anterior cervical plate and bone screw components are intended for anterior interbody screw fixation from C2-T1. The indications and contraindications of spinal instrumentation systems should be well understood by the surgeon.
The plate and bone screw components are indicated for use in the temporary stabilization of the anterior spine during the development of spinal fusions in patients with:
- degenerative disc disease (as defined by neck pain of discogenic origin with degeneration of the disc confirmed by patient history and radiographic studies)
- trauma (including fractures)
- tumors
- deformity (defined as kyphosis, lordosis, or scoliosis)
- pseudoarthrosis
- failed previous fusions
Risks of the ZEVO™ Anterior Cervical Fusion System include but are not limited to early or late loosening of any or all components and the development of new radiculopathy, myelopathy or pain, and/or tissue or nerve damage caused by improper positioning and placement of implants or instruments.

This product incorporates technology developed by Gary K. Michelson, M.D.
References
- Anterior cervical plating technique to prevent adjacent-level ossification development [2013-04-19. Dong-Ho Lee, MD, PhD, Jung-Sub Lee, MD, PhD, Jin-Seok Yi, MD, Woojin Cho, MD, PhD, Lukas P. Zebala, MD, K. Daniel Riew, MD. The Spine Journal.com]
- MEDTRONIC RECEIVES FDA CLEARANCE FOR ZEVO™ ANTERIOR CERVICAL PLATE SYSTEM [2015-05-04. Medtronic.com]
- IMPORTANT SAFETY INFORMATION for ZEVO ANTERIOR CERVICAL PLATE [Medtronic.com]
- Medtronic gets FDA clearance for ZEVO anterior cervical plate system — 5 things to know [2015.05.05. Becker’s SPINE REVIEW]